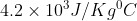Heat formula is given as
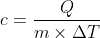
Where,
- m is the mass of the body
- C is the specific heat
- Δ T is the temperature difference
Heat formula can be applied to find the heat transfer, mass, specific heat, or temperature difference.
Heat is expressed in Joules (J).
Solved Examples
Determine the heat needed to raise a half kg of iron from 250 C to 600 C?
Solution:
Given parameters are
Mass m = 0.5 Kg,
Specific heat of iron c=
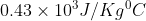
, Temperature difference
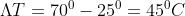
the heat formula is given by
Q = m × c × Δ T
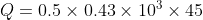
Example 2
Determine how much heat energy is lost if the water of 5 Kg mass is cooled from 60 degree c to 20 degree c.
Solution:
Given parameters are,
Mass of water is 5 Kg,
Specific heat of water C is